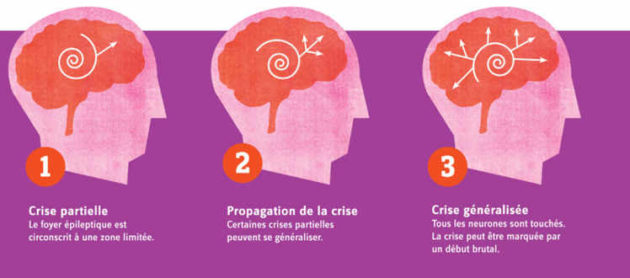
Addex Therapeutics (SIX: ADXN and Nasdaq: ADXN), a clinical-stage pharmaceutical company pioneering allosteric modulation-based drug discovery and development, today announced that its partner Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, has received FDA’s Investigational New Drug (IND) approval to begin a Phase 2a proof of concept study with the selective metabotropic glutamate type 2 (mGlu2) receptor positive allosteric modulator (PAM), JNJ-40411813 (ADX71149), in patients with epilepsy. The first patient is expected to be treated during Q2 2021.